Today, at South Ural State University, 8 international research laboratories are successfully functioning and are headed by the leading foreign scientists from the USA, Canada, Mexico, United Kingdom, France, Slovakia, and India.
Within the frameworks of the 2019 International Scientific Council, the projects of four more laboratories were approved. One of these will be the Laboratory of Magnetic Oxide Materials, the leading scientist of which will be Doctor of Sciences (Physics and Mathematics), Professor Vladimir Gudkov (Ural Federal University). Head of the Crystal Growth Laboratory of the SUSU Nanotechnology Research and Education Centre, Doctor of Sciences (Chemistry), Associate Professor Denis Vinnik shares on what research studies will be carried out at the laboratory.
– What is the goal of opening the Laboratory of Magnetic Oxide Materials at SUSU?
– The laboratory’s activity is based on the results of the research conducted earlier, and the work will be carried out within the frameworks of the formed scientific field: obtaining of hexagonal ferrites and of solid solutions based on them. The task of our laboratory is to elaborate a theory, which would allow to explain the influence that the substitution at the atomic level in the barium hexaferrite lattice on the changing of the structure, and therefore, the properties of this material. In other words, having created the theory and formed the idea on how the changes in the chemical composition influence the ultimate changes in the properties, we will be able to control and adjust the properties of the materials being obtained. The distinctive feature of the laboratory will also be the fact that one of its supervisor will be Professor Gudkov, one of the leading Russian scientists, who is a recognized world leader in studying oxide monocrystals using the method of ultrasound exposure in weak and strong magnetic fields.
– What is the peculiarity of such materials as ferrites?
– Ferrites are ferric-oxide based materials. The peculiarity of the crystalline structure of ferrites is that it has 5 different positions of ferric iron in the lattice. Namely this feature allows to modify the structure and properties of the material within quite a broad range. Our interest in barium ferrites is explained by their unique functional characteristics. The chemical stability and corrosion resistance makes these materials environmentally friendly. Low specific electrical conductivity allows to use hexaferrite magnets with high-frequency magnetic fields, what makes it promising for microelectronics. Currently, this material has big potential with regard to absorbing electromagnetic radiation in the microwave band. Hexaferrites are used in microwave technologies, as well as in data transmission, and protection against exposure to high-frequency waves.
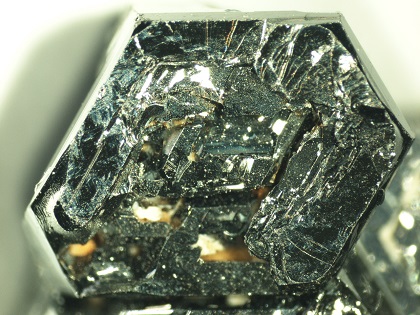

Photo: Single crystal of strontium hexaferrite; single crystals of lead germanate
– Why is barium hexaferrite being actively studied by researchers across the world?
– Hexagonal ferrites were discovered quite a while ago, in the middle of the 20th century. Originally, these were used for creating of permanent magnets. The next stage was the elaboration of the systems of information recording and storing, and in the recent 10–15 years a new trend has appeared: the focus is being made on using the microwave properties of the material (in microwave electronics, various antennae, etc.). These materials are most in demand for producing the elements of microwave electronics, and this is the global trend. Be predicting the influence of the substituting element in the structure of the material on its final microwave properties, we can make assumptions on what properties the material itself will have. Our task is, after theoretical predicting and modelling, to obtain this material, study its chemical composition and assess if we were right in our assumptions.
– How will the SUSU’s international collaboration be developing at your laboratory?
– For several years now, our group has been working with the Institute of Inorganic Chemistry at University of Stuttgart, Germany; and Lappeenranta-Lahti University of Technology, Finland. Besides that, we are trying to develop collaboration within Russia: among our partners are Lomonosov Moscow State University; Institute of Geology and Mineralogy, Siberian Branch of the Russian Academy of Sciences; Institute of Semiconductor Physics, Siberian Branch of the Russian Academy of Sciences, and other institutions. Also, within the frameworks of the laboratory’s activities, research will be held jointly with High Magnetic Field Laboratory, Germay; The Universty of Texas in Austin, USA; Moscow Institute of Physics and Technology; Ioffe Institute; and other institutions.
– Where else, except microwave electronics, can barium hexaferrite be used?
– In the context of the growing amounts of different devices and gadgets, the electromagnetic pollution is becoming an increasingly serious problem around the world. One of the potential fields of ferrites application could be their use for creation of coatings reducing or completely preventing the impact of electromagnetic radiation on different devices and living organisms.
– What will be the unique feature of the Laboratory of Magnetic Oxide Materials?
– The current global trend is the use of ferrites in microwave electronics. The number of scientific publications on this topic is rapidly growing. On the one hand, we are keeping up with the global trend, but on the other hand, we have competitive advantages in the form of the methods of obtaining ferrites as single crystals. Our advantage is that we manage to achieve greater degrees of ferric iron substitutions, and in addition, we combine the in-demand ferrites field and the obtaining of new materials in the form of high-entropy compositions (solutions that contain no less than 5 components in comparable quantities). All of that allows to vary the properties of materials within a broader rage.




