The more global a goal, the more important the result. South Ural State University researchers have set a very difficult goal for themselves – create new crystal forms of carbon with a high amount of nitrogen. In the age of the rapid development of nanotechnologies of Industry 4.0, the use of such materials in electrochemistry and electronics will allow humanity to significantly improve the components of electronic devices.
Over the last 10 years in chemistry the field of obtaining covalent organic frameworks (COF) has been rapidly developing worldwide. This field of scientific knowledge is of special interest for researchers at South Ural State University. Right now, work is being completed in three areas which are tied, one way or another, to carbon materials. The main goal remains creating a new crystal form of carbon. Dmitriy Zherebtsov, senior researcher of the Department of Materials Science and the Physiochemistry of Materials, engineer of the Nanotechnology Research and Education Center, candidate of chemical sciences, told us about the properties of carbon bonds, methods of their application, and the prospects of this field of science.
“There are three crystal forms of carbon: diamond, graphite, and fullerene. Decades ago, the possibility of the existence of different crystal forms of carbon in which the atoms are more complexly aligned was predicted theoretically.
Over the last 10-15 years, tens of various forms of covalent frameworks have been synthesized. They all differ by their open spatial architecture, created by aromatic cores, linked together by bridge groups, which overall creates a 3D mesh. The bridges used for synthesizing covalent organic frameworks (COF) are oxygen- or nitrogen-containing groups. With heat, these bonds undergo complete decomposition. If we are able to create an analogous covalent organic framework, in which the bridge groups will simply be atoms of carbon (which no one in the world has done yet), then with subsequent thermal decomposition, we can obtain a clean carbon material. Its structure inherits the structure of organic crystal, a covalent organic framework, which we obtain at room temperature,” explains Dmitriy Anatolyevich.

The open structure of carbon grids determines a number of unique physical characteristics of the material such as high conductivity, chemical inertia, and high specific surface area. This collection of characteristics makes the materials promising for application in electronics, in gas sensors, and, most of all, in electrochemical devices: batteries, capacitors, fuel elements, and solar and hydrogen cells. This will make it possible to improve the quality of electrodes and reduce the size and mass of electronic devices.
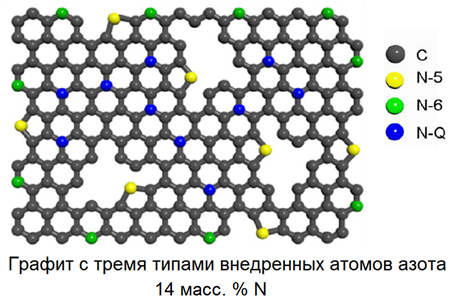
“At this time we are synthesizing a different class of carbon materials -graphites with an unusually high amount of nitrogen (up to 30% of the weight percentage of nitrogen). They have a crystal grid which does not differ from graphite. But a portion of the atoms of carbon in their structure are replaced by atoms of nitrogen. This gives this kind of material better conductivity, improves its chemical inertia in oxidation reactions on the surface of electrodes, and also improves the selective adsorption of cations of various metals or organic molecules in such nitrogen-rich materials,” says Dmitriy Zherebtsov, “Materials based on nitrogen-doped graphite are of interest for such fields of science and technology as electrochemistry and sensors able to detect concentration changes of gaseous substances or those dissolved in liquid.”
Study of new carbon materials has been in progress since 2005, and serious results have been achieved. For example, one of the most recent achievements was the growth of monocrystals and the solution of the crystal structure of two dyes which are widely used in industry, synthesized more than 50 years ago. This was onlyachieved in 2017.
.png)
.png)
One of the most important current projects is the formation of a patent, “Methods of obtaining carbon materials with a high nitrogen content.” In addition, the types of graphite with high nitrogen content obtained in 2016-2017 attracted the attention of researchers from Taiwan, Finland, and France. At this time, two applications are being put together for international Russian-Taiwanese and Russian-French grants. The international interest in this topic shows the importance of the research being done and their promise.
No less important, according to Dmitriy Zherebtsov, is the fact that it’s not necessary to have world-class financing to complete world-class research. After all, synthesis of 84 samples of nitrogen-doped graphite was completed with just 200 rubles in expenses for chemical agents (2016-2017). The researcher also told us about other areas related to obtaining graphite materials
“The main area is covalent organic frameworks. The second area is nitrogen-doped graphite, in which international work is being completed, and in which we are participating. The third area is the creation of new, polycyclic aromatic surface active substances. Many laboratories around the world are working on this, and we are able to keep up with international scientific societies. These substances also have many promising areas of application in technology: in solar elements and liquid crystals, of interest for optics and electronics.”
At this time, work with carbon materials is moving to the next higher level, the researcher believes. The complexity of the goals set requires more time, and reading the sizable stream of fresh scientific literature in this area of knowledge, mostly in English, daily.
“After accumulating a certain amount of knowledge in such hot topics as, for example, graphenes or nanotubes, the quantity of information turns to quality, and the pyramid of knowledge which you built brick by brick by reading scientific works will allow you to decide in which empty space you should set your brick to finish building this pyramid.”

In any scientific area, as Dmitriy Anatolyevich notes, it is impossible to complete all the various kinds of research alone. As such, the study of carbon materials is completed with researchers from the SUSU Faculties of Chemistry and Physics (Yekaterina Batashevich, Vycheslav Avdin, Vycheslav Yeremyashev, Kseniya Smolyakova, Denis Vinnik, Fedor Podgornov, Sergey Morozov, and others) and students of the university.




